Introduction
Citizen science flourishes especially in research fields that involve the processing of large amounts of data, such as environmental sciences, ecology, or geography (Hecker et al., 2018; Kullenberg & Kasperowski, 2016; Pettibone et al., 2017). In medical and health research, by contrast, few activities are labelled as citizen science to date. However, medical and health research has its own long-standing traditions of participation by non-scientific actors; for instance, the approaches of patient and public involvement (PPI; Baines & Regan de Bere, 2018), patient-oriented research (POR; Rouleau et al., 2018), community-based participatory research (CBPR; Wallerstein et al., 2018), or participatory health research (PHR; Wright & Kongats, 2018). Both fields—citizen science and established participatory approaches in health research—so far seem to co-exist largely disjoined from each other (Burns et al., 2021).
From a multidisciplinary citizen science perspective, the health field might look like a somewhat ordinary research field if the focus is on crowdsourcing or other formats where laypersons have a supplying role in data collection (Borda et al., 2020; Hood & Auffray, 2013; Walls et al., 2019). Here, citizen scientists might, for instance, use digital devices to record and report when they experience an allergic reaction and where they are at that moment. Or they gather concrete symptoms and their experiences with specific drugs and share these data on health social networks such as PatientsLikeMe, where professional scientists may use and analyze it for scientific purposes. In these cases, the effort of the individual participant seems manageable, even if she or he pursues these activities while being ill. Yet, what if the participants’ role is far more active? What if they are involved in all phases of the research process, including the formulation of the research question, research design, data collection, data analysis, interpretation of results, and dissemination? Especially in the context of chronic illness, participants might be ill (i.e., suffering from a chronic disease) on the one hand, but healthy enough to engage in research on the other. They then participate as patients under vulnerable circumstances, since their health condition can worsen at any time (including becoming life threatening), possibly forcing them to interrupt their engagement as citizen scientists. Thus, citizen science in health-related research may include more than ordinary formats of citizen science. It can also mean the involvement of patients as a vulnerable group, even on a high engagement level, representing extreme citizen science or participatory science in Haklay’s (2013) sense, or a patients-as-research-partners approach (Smith et al., 2019).
To investigate the implications of such a fragile context, which is rather unfamiliar for the citizen science community, as well as the benefits and challenges of such an approach, we set up a citizen science project entitled “Patient science for the investigation of rare diseases – a citizen science study on cystic fibrosis.” It was funded (2017–2020) by the German Federal Ministry of Education and Research (BMBF) within its research-funding program for citizen science. The aim of this program was to strengthen the field of citizen science through selected projects that were generally expected to address a socially-relevant scientific issue and advance citizen science in an innovative-methodological way (BMBF, 2016). Given that the funding context was citizen science in general (and not clinical trials or health research where the above-mentioned other participatory approaches are common), we aimed for a fresh look from the perspective of the growing citizen science community in Germany.
We chose rare diseases as the object of investigation because there is a general scarcity of research in this field. In fact, due to their rarity, many research actors see rare diseases as anything but an attractive research field, for both medical and economic reasons (NAMSE, 2013). Thus, by making that choice, we expected our citizen science project to have the potential to contribute profoundly not only to the research field but also to the improvement of the living situation of those affected by the disease.
Among rare diseases, cystic fibrosis was chosen for pragmatic reasons. Since it is a rather common rare disease (about 8,000 people are affected in Germany, according to the German Cystic Fibrosis Association), there are good and professional self-help structures in Germany, which the patient science project could make use of. For this reason, the German Cystic Fibrosis Association was included as partner organization within the consortium of the Fraunhofer Institute for Systems and Innovation Research, University Hospital Frankfurt/Main, and Ostfalia University of Applied Science. Cystic fibrosis (CF) is a monogenetic lethal metabolic disease that produces a very thick mucus in many organs of the body, especially the lungs and the digestive tract. It can be treated, but is still incurable. Patients need to take medication throughout their lives, inhale regularly, and perform special breathing therapies and physiotherapeutic exercises on a daily basis. CF severely influences the lives of patients and their relatives in many ways.
Following the considerations outlined above, the patient science project pursued a far-reaching participatory approach that entailed co-creation (as defined by Shirk et al., 2012) in all phases of the research process. Professional scientists and citizen scientists (or patient scientists, as we call them) jointly planned, implemented, and evaluated a scientific study on a rare disease from which the patient scientists themselves suffer. At the time of funding application, the precise research topic and question had not yet been determined, for good reasons, since this was supposed to be carried out jointly with the patient scientists. It had simply been defined that the project’s first aim was to investigate one or several essential problems related to the disease (CF) in the everyday life of patients and their relatives, and thus to contribute to solving the problem(s) and improving their living situation. The second predefined aim of the project was to identify the benefits and challenges of patient science as a citizen science approach involving chronically ill people.
In this article, we present our findings on the second aim. Therefore, our intention is not to present and discuss the empirical findings regarding the everyday problems of patients and their relatives (this is covered in Gardecki et al., 2020, and Gardecki et al., forthcoming). Instead, we aim to present our co-creative participatory approach and reflect on the involvement of chronically ill people as co-researchers in citizen science. To begin with, we describe the design and process of our project in more detail to create a reference point for the following sections. We then put the patient science approach in relation to other participatory approaches in medical and health research. Subsequently, we elaborate on the implications of involving chronically ill people in the research process before we finally present and discuss the benefits and challenges of patient science.
The Patient Science Project Using the Example of Cystic Fibrosis
To get the envisaged co-creation process started, a transdisciplinary research team was set up, including twelve patient scientists and eight professional scientists. In this article, “research team” refers to this team of co-researchers, including professional and patient scientists. The patient scientists were recruited by practitioners from University Hospital Frankfurt during routine health care appointments. The first patient scientist was recruited (in the same way) even before the start of the project so that he could help advise on the recruitment process. Prerequisites for recruitment were: a reasonable state of health, compatibility with therapy obligations, and motivation to actively participate in a research project. Selection criteria were applied to ensure that the participants varied in age (range 18 to 50 years, mean 30, median 24), gender (7 female, 5 male), and occupational background (2 pupils, 5 students, 3 employees, 2 invalidity pensioners). Among the patient scientists were ten patients directly affected by CF and two relatives, namely parents of an affected child (1 mother, student; 1 father, employee). They all received an hourly wage for any time spent working on the project, regardless of if they were working on research, organizing, training, dissemination, traveling, or other project activities. The payment method and amount were based on a standard work contract comparable to student research assistants. The group of professional scientists (5 female, 3 male) included four social and health scientists, two physicians, one psychologist, and one health economist from all of the consortium’s partner organizations. In order to enable the patient scientists to act as co-researchers, they received training, whenever needed, via lectures and seminars from the professional scientists. Topics included research design, basics of empirical social research, questionnaire construction, and statistical data analysis.
As explicitly stated from the start, this project aimed to collaborate within the research team on a co-equal level (“eye level”), assigning the involved professional expertise the same appreciation and importance as the so-called “lay expertise” (Epstein, 1995; Prior, 2003) of the participating patients and relatives. This expertise, which we call patient expertise, has been built up by intensively dealing with the chronic disease for a long time. In other contexts, the same subject has been referred to as “experiential knowledge of patients” (Caron-Flinterman et al., 2005) or “lived experience” of a specific health condition (Brett et al., 2014; Williams et al., 2020). Building on a long tradition of the sociology of knowledge and expertise within Science (and Technology) Studies (Collins & Evans, 2002), we prefer to use the term “expertise” to illustrate the equal importance of different kinds of knowledge involved (Callon & Rabeharisoa, 2003). Since such expertise of non-scientific people, normally deemed non-experts or laypersons, is not certified or recognized by any academic or social institution, Collins and Evans (2002) call it “non-certified expertise” in contrast to the “certified” expertise of professional scientists. Particularly in health and medicine related fields, such a notion of lay knowledge or lay expertise has long been common, precisely with regard to patients who have intensively dealt with a chronic disease for many years (Brown et al., 2004; Callon & Rabeharisoa, 2003; Epstein, 1995; Johansson, 2014; Prior, 2003).
Unfortunately, the research team was never able to meet face-to-face in its entirety because some of the patient scientists carry specific germs (pseudomonas) while others do not, and these groups should not mix due to the risk of infection. Thus, long before the COVID-19 pandemic, the research team was forced to primarily meet and communicate online, or to meet face-to-face, but then in subgroups and different rooms, or to exclude one of the two subgroups of patient scientists. This applies to the whole research process described hereafter.
The first step was to define the specific research question and research design. This was done by a co-creation process consisting of two full-day workshops (facilitated by two external moderators) where several topics with a need for research and the corresponding epistemic interest were identified. These topics included: 1) the development of practical guidelines on how CF patients and their relatives could individually optimize their personal balance between medical recommendations and requirements for everyday life on the one hand, and their perceived quality of life on the other hand (CF-life-balance); 2) the measurement of indirect or hidden financial costs that a life with CF may cause (e.g., old-age poverty due to part-time employment); 3) the development of a concept about how CF patients can be supported in their jobs or academic studies; 4) the development of a concept about the challenging transition from children’s to adult’s CF outpatient clinics; 5) the identification of novel (formerly unknown) side effects and interactions between various drugs.
Ad-hoc voting among professional and patient scientists resulted in simple majorities for the first two research topics. Nevertheless, all five research topics were evaluated according to the following criteria: relevance to everyday life of CF patients; technical feasibility (including available competencies, financial resources, and time frame); relevance to the state of CF research and the scientific CF discourse; potential wider societal impacts; added value by following a citizen science approach; potential impact on the scientific literacy of all participants. This evaluation also resulted in a preference for the first two topics.
After a long and intense discussion, the research team decided to address the first two research topics (CF-life-balance and indirect CF costs). This allowed us to combine key aspects of both topics, but also required a reduction of the original ideas. It was finally agreed that the aim of the upcoming patient science study was to, for the first time in Germany, systematically map and measure the typical and most important everyday problems of CF patients and their relatives (including the financial situation concerning CF) and to analyze the resulting needs for support and guidance (for a good CF-life-balance). Accordingly, the key research question was the following: What are the typical and most important everyday problems of CF patients and their relatives (mostly parents of CF-affected children)? After this decision was made, the research design was quickly determined: a standardized online survey of the German CF community.
In this first phase of the research process (see Figure 1), the main contribution of the patient scientists was to gather and prioritize the research needs and topics and then to evaluate them regarding the relevance to everyday life of CF patients. The main contribution of the professional scientists was to propose methodology options for each research topic and to evaluate them regarding all other criteria, such as technical feasibility.
It should be emphasized that this process of defining the research question and design was open-ended and could have easily led to another research question and research design. Beforehand, during funding application, merely a phase of empirical data collection and data analysis had been predefined. If another research topic had been prioritized, another research design (possibly one with qualitative or mixed methods) would have been chosen. After this first phase of the research process, the first year of project duration (out of three) was nearly over. That might illustrate the intensity and necessary effort of both setting up the research team and defining the research design.
In the next phase, the questionnaire for the standardized online survey was developed, again within a co-creation process. At first, about 30 relevant themes concerning everyday problems of CF patients were jointly gathered in several brainstorming sessions. They were then clustered into five main fields: 1) professional care and treatment; 2) occupation, including school, university education, training, and employment; 3) living and housekeeping; 4) social life and leisure; and 5) mobility and travel. For each field, potential questions for the survey were collected, structured, reviewed for their relevance to the overarching research interest, and excluded where appropriate. Finally, the remaining set of questions was operationalized. In retrospect, both professional and patient scientists agree that this questionnaire would have been very different if it had been developed by professional researchers alone.
Despite numerous revisions, the final questionnaire was still extraordinarily long, encompassing 271 items (although not applicable for every respondent). From a conventional academic perspective, this is far too long; it resulted in an average processing time of 60 minutes, which is usually considered unacceptable. The research team considered alternative options, such as allowing respondents to freely choose how many parts of the questionnaire they would cover or offering a small financial reward for those who complete the entire questionnaire. However, since this survey was meant as a scientific study from the CF community for the CF community, and since the research team considered this a unique opportunity to gather highly-relevant data, the joint decision was taken to leave the questionnaire at that length. This again illustrates the distinctive effects of the co-creative collaboration in this project.
The final questionnaire was structured as follows (see Figure 2): In a first part, sociodemographic data such as age, gender, and living situation were queried (part A). Next, the validated and well-established health-related quality of life questionnaire CFQ-R (“Cystic Fibrosis Questionnaire – revised version”) (Wenninger et al., 2003) was included in order to analyze correlations between CF-related quality of life scales and everyday problems (part B). The third part covered professional care and treatment, specifically the reachability of CF outpatient clinics, the availability and competence of specialists, and the financial situation concerning CF (part C). The next part, on occupation, asked for the thoughtfulness of teachers, classmates, or colleagues, and the difficulties of carrying out therapy in public places, absenteeism in school or employment, and additional related topics (part D). The fifth part covered living and housekeeping issues, including the distance between home and the CF outpatient clinic, and domestic hygiene to prevent infections (part E). The following section focused on social life and leisure, such as support from family or friends, or restrictions with regard to leisure activities due to CF (part F). Next, the questionnaire focused on mobility and travel, covering limitations in everyday mobility due to CF as well as restrictions regarding holidays (part G). Finally, the respondents were asked to prioritize everyday problems by indicating which of 19 specified problems were most important and required the most support and guidance. They were also asked to make suggestions on what could help with these problems (part H).
The main contribution of the patient scientists in this phase of the research process (see Figure 1) was to gather and cluster everyday problems, formulate appropriate questions for the questionnaire, and prioritize them for each field. The main contribution of the professional scientists was to operationalize the questions (making sure that they were valid regarding the epistemic interest at hand), to technically implement the questionnaire as an online survey, and to evaluate the pre-test.
After the survey was approved by the Ethics Committee of the Goethe University Frankfurt, the questionnaire was implemented as an online survey and promoted especially by the German Cystic Fibrosis Association using its membership journal, mailing lists, website, and social media. In this third phase of the research process, the main contribution of the patient scientists was to compose text pieces for the different calls for participation distributed by diverse communication channels (such as social media postings), and to give promotional talks (together with professional scientists) at public events such as the annual CF self-help conference in Germany. Apart from these talks, the professional scientists were mainly responsible for the technical distribution of the call for participation on all communication channels used.
The online survey was open for participation for a period of three months in Summer 2019 (July to September). Consequently, 902 CF-affected persons completed the whole questionnaire. 51% were patients (ages 14 years and older), 49% were relatives, namely parents of a child with CF (children aged 13 years and younger). This response rate can be seen as a great success since it means that more than 10% of the estimated 8,000 CF-affected persons in Germany could be included in the study. The total of 902 completed questionnaires is also remarkable, particularly when considering the length of the questionnaire. After closing the online survey, the second year of the (three years long) project duration was over.
In the next phase of the research process, the immense amount of survey data had to be statistically analyzed and interpreted. After professional data preparation and the first run of statistical data analysis procedures (and after some training of the patient scientists in how to read statistical output tables), an analysis template for the patient scientists was developed to support them in: 1) summarizing the descriptive statistics; 2) identifying key findings; 3) highlighting personal insights and interpretations; and 4) suggesting further steps of statistical data analysis (such as correlation analysis). All patient scientists involved in that phase were responsible for a specific part of the questionnaire, whereas the professional scientists were responsible for carrying out the statistical procedures. In addition, professional scientists also highlighted and interpreted findings, especially in exchange with the patient scientists.
The task of the final phase in the research process was the dissemination and exploitation of the results. One important step here was to organize an online symposium where the findings were presented and discussed with invited experts from the CF self-help community, CF research, CF professional care, and the general (not CF-related) citizen science community. Again, the results were partly presented by patient scientists and partly by professional scientists. Likewise, this holds true for other oral presentations at conferences, also during the course of the project (such as CF self-help meetings and conferences, or the German Citizen Science Forum).
Regarding the survey results, we have consistently received feedback during our oral dissemination activities from the CF community (self-help, research, and professional care) that not only were the “right/relevant” questions asked in our comprehensive questionnaire, but that the patient science project has “finally” created scientific-empirical evidence for many problems in everyday life with CF, meaning that the CF community now has access to “data instead of anecdotes.” According to the community’s feedback, these data have a high usability for many activities in CF-related health politics, patient care, and self-help.
As well as the symposium, our general dissemination strategy followed a double tracked approach: One track aimed at addressing the CF community (self-help, research, and professional care). Here, both professional and patient scientists jointly selected the most important findings of the online survey and prepared them for a comprehensive and easily understandable brochure. It was the patient scientists who made a special effort to ensure that the brochure also entailed helpful practical information for CF-affected people. Of course, they are co-authors of this publication (although many of the patient scientists chose to stay anonymous). Moreover, a scientific journal article is in preparation (Gardecki et al., forthcoming).
The other track aimed at addressing the communities of citizen science and participatory research. The key results relevant here are presented in this article and refer to the reflection of the participatory approach explored in our project. They are based on a self-evaluation process throughout the project, including an annual standardized survey among all participants and a qualitative self-reflection questionnaire filled out by several professional and patient scientists at the end of the project. Accordingly, one of the patient scientists is co-author of this article (SP). In addition, we developed a practical document (in German language only) that summarizes our practical experiences and insights and formulates recommendations for future patient science projects (Heyen et al., 2021).
Throughout its duration, the project received relatively high interest from German media, including several articles in national daily newspapers and popular magazines as well as multiple radio podcasts. One public radio station even decided to accompany the project journalistically and produced two half-hour podcasts about the project’s aims, process, and results. After being trained in how to deal with journalists and media requests, patient scientists were heavily involved in these public relations activities, mainly by giving interviews.
The Relationship to Other Participatory Approaches in Health Research
As indicated in the introduction, medical and health research has its own (and rather old) traditions of participation by laypersons or the public, being reflected in a vast amount of literature (Brett et al., 2014; Domecq et al., 2014; Greenhalgh et al., 2019). One of the internationally well-established key terms here is patient and public involvement (PPI) (Baines & Regan de Bere, 2018). The particularly prominent and influential British initiative INVOLVE defines this “public involvement in research as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them”, whereas “public” includes “patients, potential patients, carers and people who use health and social care services as well as people from organisations that represent people who use services” (INVOLVE, 2012, p. 6; emphasis in the original).
In other parts of the world, similar research activities and practices are referred to as patient-oriented research, for example in Canada (Rouleau et al., 2018), or as patient engagement in research, especially by the prominent Patient-Centered Outcomes Research Institute (PCORI) in the United States (Domecq et al., 2014). However, such terms—quite similar to citizen science—are umbrella terms, comprising a huge diversity of concepts and approaches, including very different levels of patient or public engagement (Williams et al., 2020). According to INVOLVE, for instance, PPI includes “working with research funders to prioritise research, offering advice as members of a project steering group, commenting on and developing research materials and undertaking interviews with research participants” (INVOLVE, 2012, p. 6).
Similar to citizen science, there have been many attempts to distinguish and classify these diverse concepts and approaches with respect to the level of engagement. INVOLVE (2012), for example, distinguishes between consultation, collaboration, co-production, and user-controlled research. Goodman and Sanders Thompson (2017) more generally distinguish between non-participation, symbolic participation, and engaged participation, and define subgroups for each category. With regard to engaged participation, subgroups include collaboration, patient-centered research, and community-based participatory research (CBPR).
As should have become clear in the previous section, the patient science project belongs to categories such as “co-production” (INVOLVE, 2012) or “engaged participation” (Goodman & Sanders Thompson, 2017). However, in contrast to many studies in PPI and related contexts, the patient science project did not follow the logic of clinical trials (Gaasterland et al., 2018; Mader et al., 2018; Staniszewska et al., 2012). And although the research team decided to conduct a survey through an open-ended process, the project in its entirety went beyond the mere collaborative development of a questionnaire (Mes et al., 2019) or so-called patient-reported outcome measures (PROM) (Staniszewska et al., 2012).
The heterogeneity of approaches within the categories such as “co-production” or “engaged participation” is still high (Greenhalgh et al., 2019; Williams et al., 2020). There are two in particular that seem to be similar to the approach we used in the patient science project—namely the community-based participatory research (CBPR) (Wallerstein et al., 2018) and the participatory health research (PHR) (Wright & Kongats, 2018) approach. To further sharpen the profile of the patient science approach and to illustrate its citizen science character, we briefly reflect on some differences compared to PHR, which shares many principles and aspects with CBPR (von Unger, 2012).
According to the International Collaboration for Participatory Health Research (ICPHR), the “goal of PHR is to maximize the participation of those whose life or work is the subject of the research in all stages of the research process … Such participation is the core, defining principle of PHR, setting this type of research apart from other approaches in the health field. Research is not done ‘on’ people as passive subjects providing ‘data’ but ‘with’ them to provide relevant information for improving their lives” (ICPHR, 2013, p. 6; emphasis added).
Whereas the patient science approach shares with PHR the ambition to achieve the highest possible level of participation in all phases of the research process, it does not aim primarily—nor directly—at the improvement of participants’ lives. Rather, patient science pursues epistemic goals in the first place, although with the ambition of having socioeconomic or political impacts regarding the people affected by the disease in focus. PHR, in contrast, stands in the tradition of action research (Reason & Bradbury, 2008) and is, therefore, more strongly aimed at the continuous transformation of specific living conditions or practices.
Moreover, PHR is closer to the public health sector and thus focuses on socially disadvantaged people in order to mitigate the effects of social determinants of health (such as low income or low educational level) and to foster equal health opportunities. Patient science instead focuses more on people disadvantaged in terms of health: people with chronic diseases or other health problems, regardless of their socio-economic status. Another difference between PHR and patient science is that PHR basically follows a setting approach (Poland et al., 1999), focusing on local conditions and producing local knowledge and local evidence (ICPHR, 2013). Accordingly, PHR strives to involve all actors concerned in the specific setting, being it residents of the neighborhood, health care professionals, representatives of civil society institutions, policy makers, or other stakeholders. This may include laypersons, but not necessarily. Patient science, on the other hand, explicitly aims at the participation of laypersons or non-professionals, namely patients or people directly affected by a specific disease.
In addition, these people are not, as in PHR, involved just because they are concerned by the aspired transformation and therefore should have a say but because their perspective and expertise as patients is needed in the epistemic process. The same holds true for health care professionals. They are not asked to participate because they are affected players within a specific setting (as in PHR), but because their health or medical expertise is needed. Apart from that, they could easily be the (co-)initiator and (co-)organizer of the whole patient science process, so that they are themselves the ones who invite the patients to participate.
In sum, whereas PHR is more about the empowerment of the (lay and professional) participants with regard to (their) specific health-related living or working conditions and problems, patient science is more about the empowerment of the (lay) participants with regard to general knowledge production (being the main function of science). That is why patient science can rightly be labelled as citizen science. Against the background of the entire field of citizen science with its main research areas in environmental sciences, ecology, or geography (Hecker et al., 2018; Kullenberg & Kasperowski, 2016; Pettibone et al., 2017), the patient science approach can be further characterized by the following features. First, it has a specific thematic focus on health and medicine. Second, it involves patients as chronically ill people or people affected by a specific disease, such as relatives of patients. Third, it pursues a far-reaching participatory approach, namely co-creation in all phases of the research process. Finally, it systematically uses the specific expertise of the (normally deemed lay) participants: the patient expertise on everyday life and coping with the relevant disease.
Apart from the established participatory approaches in medical and health research discussed above, there are two other, rather new terms worth mentioning in this context. One is participatory medicine and the other is patient-led research. Participatory medicine is understood as “a cooperative model of health care that encourages, supports, and expects active involvement by all parties (clinicians, patients, caregivers, administrators, payers, and communities) in the prevention, management, and treatment of disease and disability and the promotion of health” (Gruman & Smith, 2009). Thus, participatory medicine is primarily targeted on health care rather than research (deBronkart, 2018). Where it also relates to research, it is more about generating evidence regarding the impacts and outcomes of participatory medicine (Dyson, 2009; Green, 2009; Palmer, 2020) or, following a systems (bio)medicine approach, about a crowdsourcing type of research using personal health data clouds (Hood & Auffray, 2013). It is obvious that these practices differ from what has been described here as patient science.
Patient-led research, also called participant-led research, has been introduced as a term to describe a new kind of research that usually takes place outside of institutional science and established frameworks (Vayena et al., 2016; Wicks, 2018). Although there are attempts to institutionalize some (other) sort of research led by patients (Greenhalgh, 2019; Mader et al., 2018), the term patient-led research typically indicates research activities “in the wild” (Callon & Rabeharisoa, 2003), representing a form of “uninvited” participation (Wehling, 2012; Wynne, 2007). Here, individuals or patient groups actually lead and self-organize the research, often by using online social media or platforms. A recent example is the Patient-Led Research Collaborative which conducted the first research on Long-COVID experiences and symptoms (McCorkell et al., 2021). Patient science, in contrast, is not self-initiated or self-organized by patients; it takes place in an institutionalized setting and therefore represents a form of invited participation (Wehling, 2012; Wynne, 2007).
Some Implications of Involving Chronically Ill People
To involve chronically ill people in all phases of the research process has both general and disease-specific implications. First, it means involving a specific expertise, namely the patient expertise, which is highly valuable for the production of scientific knowledge. At the same time, it needs to be appreciated that people becoming patient scientists in order to investigate a topic related to their illness are always directly affected by the content of the study. For patient scientists, the research process entails the challenge of abstracting from their subjective view of being personally affected. Therefore, a balance between the use of personal experience and a pursued objectivity is needed.
Being directly affected by the research topic (or not) can also imply a difference in motivation for participation between the professional scientists and the patient scientists. With regard to the latter, an especially high intrinsic motivation can be assumed, since there are only a few motivating factors as strong as improving one’s own health or the health of other people suffering from the same chronic disease. This was also the case for the patient scientists in our project (all following quotes from patient scientists have been drawn from interviews, either by external journalists in the context of public relations activities or for the internal purpose of self-evaluation and reflection).
“I was thinking, maybe I can somehow make a difference, maybe I can contribute to research, not only for my family, my child, but also for the whole group of people affected by CF, whether they are directly affected or indirectly affected, such as parents or relatives.” (Patient Scientist 1)
“For me, it was the very first time to see CF patients other than myself. I went there by train, and I was totally excited for the entire half hour, just because I’ve never been so involved with CF before, and that’s totally new to be confronted with it like that. … It’s great to meet people who have the same problem, to share problems you have and get a different perspective on CF than the one I’ve had for 20 years now.” (Patient Scientist 2)
Thus, being affected by the research topic has the potential to inspire many people for a citizen science project in the health sector. Nevertheless, the patient scientists’ extrinsic motivation should also be served, for instance, as in our project, through fair payment for their engagement (cf. Smith et al., 2019).
If some professional scientists within the research team at the same time work as health care providers, it is important that both the health care providers and the patient scientists are sensitive to their different roles. Instead of “caretaker” and “cared for,” patients and health care providers meet each other as co-researchers within a team of (professional and patient) scientists with their respective expertise on a co-equal level. In our project, the patient scientists experienced this role change as follows.
“Having the opportunity to do a bit of scientific work—I always thought, oh God, research and science, that’s all rather dry—but I was proven wrong, it was great fun!” (Patient Scientist 1)
“That’s a pretty positive broadening of your horizon. It’s a new situation, right? For example, to work with doctors whom I normally meet as a patient in the outpatient clinic or hospital. The change of perspective was certainly new for both sides, but in my perception, it did not pose any difficulty. … Valuable for me was also the informal exchange beyond the normal medical consultation, like the conversations with the doctors during the workshops or during the breaks with a cup of coffee. Such a possibility is rarely available in the outpatient clinic.” (Patient Scientist 3)
“That’s something special that you don’t have this hierarchy difference but are completely at eye level.” (Patient Scientist 4)
The attitude of meeting each other at “eye level” quickly leads to patient empowerment. It includes equality based on reciprocity with open communication between equal partners in order to discuss and complement each other’s gaps in knowledge or experience. In a collaborative process, it is then possible to decide who is best suited for a specific task within the research project. In this sense, co-equal level (or “eye level”) means that the patient expertise is perceived and recognized as an essential, necessary, and valuable resource for the research process. The patient scientists in our project perceived this attitude as follows:
“I noticed right from the start that there are two groups, the patients and the professional scientists, that was definitely noticeable. However, it was not noticeable in the sense that one was telling the other something, but rather that there are simply two groups of experts and that the project only works in a joint exchange. And I see that as eye level.” (Patient Scientist 5)
“That was really at eye level. … It was communicated very clearly by all professional scientists that we, as patients or as relatives, are simply experts for this disease, because we have to cope with everyday life with this disease, the doctors don’t have to do that. … Professional and patient scientists did not contribute identical skills to this project. And that was precisely what was special about it: to work together with different qualifications. The professional scientists who are the specialists in methodology etc. and the patient scientists who are the experts in living with CF. … The collaboration with the professional scientists worked out well: They enabled us to pass on our contents using their tools. I felt that my ideas and questions were taken seriously at all times.” (Patient Scientist 1)
Chronically ill people with a good state of health are often employed and therefore have little time to work on a highly demanding research project, as we know from citizen science and other forms of participatory research. Severely affected people, on the other hand, may have more time due to unemployment, but have a higher treatment burden and typically a lower capacity to intensively engage in a project. This reveals a dilemma regarding recruiting suitable participants. When selecting patients for their co-researcher role, one must make sure they are stable enough to work actively within the project and that they still have enough time to integrate the project activities into their daily life in addition to their disease management and treatment plan.
In general, duties related to managing the disease as well as a lack of physical resilience on the part of the patient scientists may affect the entire project management and implementation. Due to frequent infections, progressive deterioration, hospital or rehabilitation stays, high treatment burden in everyday life, and concomitant illnesses, some patient scientists may often be absent, sometimes unpredictably, which makes it more difficult to schedule and achieve predefined (interim) project goals. This was especially true in our project. For this reason, project management requires a high degree of flexibility and coordination effort, and, at the same time, a forward planning approach that takes the limited resilience of the patient scientists into account.
Moreover, depending on the individual characteristics of the chronic disease in focus, there may be some restrictions that require, from a medical perspective, an adjustment of the collaboration within the research team. For example, a potential mutual danger of infection among the patient scientists, as was the case in our project, may affect the feasibility of face-to-face contact, requiring the research team to switch to digital communication tools or split into subgroups. Furthermore, some diseases require intensive treatment (e.g., every two hours), which needs to be considered when planning project meetings. In addition, the potentially negative influence of the disease on energy levels, the ability to concentrate for extended periods, or mobility must be considered for the entire research process, including individual meetings. The patient scientists in our project explain in this regard:
“Since CF is unfortunately not yet curable, the disease accompanies everyday life and thus the process and is always present while the project is running. … One needs strength and energy to be able to participate. … CF patients may feel so bad that they are not able to work and we have to take care that the patient scientists are not robbed of energy by the effort of the project, since they actually need their energy for the therapy.” (Patient Scientist 1)
“I have noticed that my own resilience is not that high. After an hour at the computer I am exhausted.” (Patient Scientist 3)
Chronic diseases might also lead to mental health issues influencing motivation and performance (Quittner et al., 2014). And under certain circumstances, the risk of severe worsening of the patient scientists’ condition or even a sudden death in the course of the project must be taken into consideration, which may affect the cohesion and motivation of the research team. All these framework conditions may create a rather fragile context when working with chronically ill people.
Benefits and Challenges of Patient Science
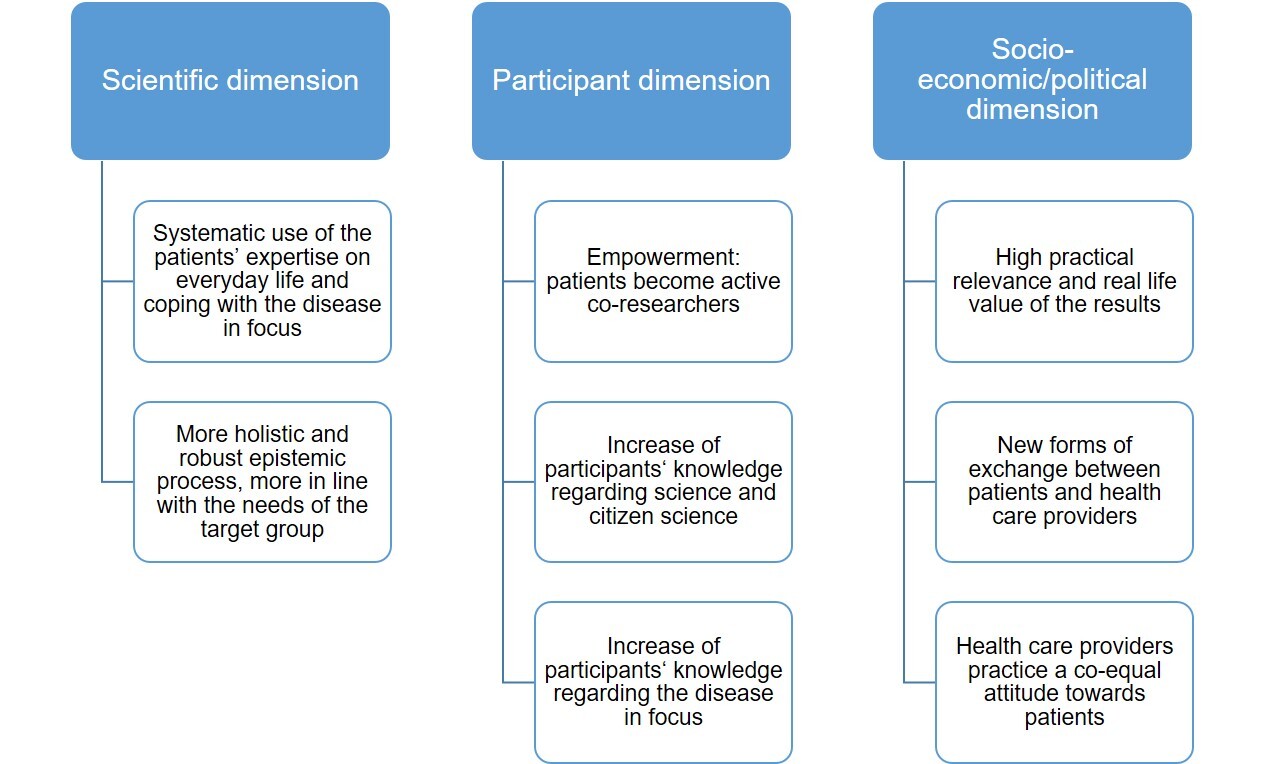
Patient science, as it has been described in this article, entails both benefits and challenges. These need to be borne in mind when conceptualizing and carrying out future patient science projects in order to maximize the benefits, mitigate the challenges and consider the limitations of the format (cf. Brett et al., 2014; Oliver et al., 2019). The benefits of the patient science approach (see Figure 3) can be clustered along the three dimensions of the citizen science evaluation framework by Kieslinger et al. (2018), namely the scientific, the participant, and the socio-ecological/economic dimension.
With regard to the scientific dimension, benefits include the systematic use of a kind of knowledge that is normally not present in research: the patient expertise on everyday life and coping with the disease (cf. Caron-Flinterman et al., 2005). In our view, drawing on this expertise fundamentally changes the entire epistemic process (including the definition of the research topic itself), as it deviates from established roles and responsibilities. It makes research more holistic, more robust, and, with regard to the target group, more appropriate to their real-life needs (cf. Russell et al., 2020).
Concerning the participant dimension, patient science gives patients and their relatives a voice in research and changes their role from being passive objects of research to being active participants, thereby empowering them. Their involvement also leads to profound learning on two levels: the participants are able to extend not only their knowledge of science in general (cf. Russell et al., 2020), and citizen science in particular, but also their knowledge of the disease in focus.
On the socioeconomic or, being more applicable here, sociopolitical dimension, the patient science approach ensures that research results are of practical relevance and real-life value—an impact that has been reported with regard to other participatory approaches in health research as well (Brett et al., 2014; INVOLVE, 2012; Russell et al., 2020; Staniszewska et al., 2012). Apart from that, it leads to new forms of dialogue and exchanges between patients and health care providers as well as among patients themselves. Finally, patient science extends the perspective of health care providers as it requires them to view their field of expertise from the real-life needs of patients and to interact with patients on an equal footing as scientists, with each group bringing a unique set of skills and expertise to the research process. If these are meaningfully combined, better and more relevant research outcomes can be generated.
Challenges of the patient science approach include finding a balance between giving as much responsibility to the patient scientists as possible while providing as much support as necessary. This will vary from group to group as well as between individuals. In addition to their knowledge of living with a certain condition and having acquired a certain degree of clinical understanding, patient scientists contribute a wide range of other skills to a research project, such as those acquired during their education or professional life. Thus, individual patient scientists start off at different levels and possess different sets of resources (skills, time, and more) to contribute to the research process. As with other teams, the collective challenge is therefore to allocate roles and training in such a way that everyone is able to best contribute their specific expertise and receive the support they require (cf. Brett et al., 2014).
As already indicated in the previous section, the distribution of tasks also needs to consider that patient scientists are affected by their illness by varying degrees. Thus, motivation, engagement, and capacity to contribute to a research project vary between individuals and throughout the course of the project. Deteriorations can occur unexpectedly and wreak havoc on pre-planned contributions. This may include longer periods of sickness at home or hospital stays, with the possibility of the worst-case event: the death of a patient scientist. These unforeseen deteriorations not only mean that others have to step in and take over tasks, but can also have a severe emotional toll on the rest of the team.
While collaboration between professional and patient scientists on a co-equal level appears to be common sense for a patient science project to succeed, it actually requires conscious efforts and the creation of favorable framework conditions (cf. Brett et al., 2014; Gaasterland et al., 2018; INVOLVE, 2012; Russell et al., 2020). As patient science upheaves traditional roles, it entails a risk of reverting to these roles if appropriate measures are not implemented. These measures may include regular meetings to ensure that all information is shared openly, consensus on values such as transparency, trust, respect, and mutual appreciation, or a division of responsibilities to allow professional and patient scientists to contribute where they are most knowledgeable. Whatever measures are put into place, they all require coordination efforts that far exceed “regular” research projects (cf. Brett et al., 2014; Goodman & Sanders Thompson, 2017).
A particular feature of patient science is that professional scientists are often also practicing health care professionals. However, a dual role of professional and patient scientist being in a patient-physician relationship as well as collaborating as co-researchers can be difficult to navigate because the patient-physician relationship usually entails a strong power asymmetry, which might not be helpful for co-equal research collaboration. If it is to be attempted, a continuous reflection of roles and expectation management is needed. At the beginning of the project, roles should be clarified in a communicative process, including the question of how the research team would determine if the collaboration was no longer on a co-equal level. Throughout the research process, a re-verification and evaluation of roles is advisable, for instance, by using (anonymous) surveys and regular reflection meetings, or by assigning co-researchers from each group the task of keeping an eye on this aspect and intervening when a co-equal level is no longer present. Moreover, an explicit naming of the two different settings (collaboration in research vs. clinical care) helps professional and patient scientists to clearly separate their two roles respectively.
Finally, where patient scientists are immunocompromised, the risk of infection—both from other patient scientists and from professional scientists—needs to be considered. This may mean that face-to-face meetings are not feasible, can only be held among a small number of participants, or need to follow strict hygiene regulations. This can hinder the development of shared identity and consequently impede commitment to the project and, ultimately, progress. To some extent, this challenge can be addressed through the use of alternative meeting formats, such as video or teleconferences, instead of text-based communication.
Conclusion
Involving chronically ill people as patient scientists in the research process entails both significant advantages and challenges. How easy or difficult the challenges are to overcome (cf. Brett et al., 2014; INVOLVE, 2012; Oliver et al., 2019; Russell et al., 2020) depends to a large extent on the framework conditions of the particular patient science project. Much still needs to be learned about which framework structures can best empower patients to actively engage in all phases of the research process, what can best encourage their responsibility for and commitment to the project, how best to achieve a communication and collaboration form on a co-equal level, and how to make the best possible use of the different resources and competencies of all co-researchers involved.
An inner attitude of being equal research partners with respective competencies, a sensitivity for the new role that patients take on in this special format, and, above all, the creation of a joint understanding of the patient science approach and how to implement it within the research process seems to be helpful in all cases (adding to Read & Maslin-Prothero, 2011). Moreover, a successful collaboration between professional and patient scientists requires a dynamic working atmosphere with transparent and reciprocal communication from the very beginning. This implies the determination of responsibilities, reliable commitment of the participants, the clarification of various tasks and goals, and the development and agreement of common rules for the co-working process (cf. Brett et al., 2014; Goodman & Sanders Thompson, 2017).
In our view, the most important added value of the patient science approach is the systematic use of the patients’ expertise in all phases of the research process. Only then can the key benefits—such as making the epistemic process more holistic, more robust, and more appropriate to the target group’s real-life needs, and generating results of high practical relevance and real-life value—be realized. At the same time, this points to a clear limitation of the approach regarding projects that would not benefit much from the unique expertise of patient scientists. This could be relevant where patients are involved primarily to ensure participation of this group, but might not be able to contribute anything novel or unique to the research topic or process. In short, the additional effort needed to successfully run a patient science project is only justified if the patient expertise is expected to make an epistemic difference.
Ethics and Consent
At the beginning of the patient science project, the Ethics Committee of the Goethe University Frankfurt, Germany, gave its oral approval to commence the project as planned (without commenting on the recruitment criteria or the payment of the patient scientists) and recommended applying for formal approval, not before the concrete study design is finalized. Accordingly, (only) the conducted survey on the typical and most important everyday problems of CF patients and their relatives was formally approved by the Ethics Committee (reference number 19-229).
Acknowledgements
The authors greatly acknowledge the valuable contributions of their co-researchers in the patient science project: Melina M., Paulina M., Katja Wecke, and eight other patient scientists who chose to stay anonymous, as well as Nicole Brkic. In addition, they would like to thank Sarah Weschke and unknown reviewers for their helpful comments and suggestions on earlier versions of this article.
Funding Information
This article is based on research funded by the German Federal Ministry of Education and Research (BMBF; project ‘Patient Science for the Investigation of Rare Diseases – a Citizen Science Study on Cystic Fibrosis’; Grant Number 01BF1707A/B/C).